What Does The Total Thermal Energy Of A System Depend On?
What does the total thermal energy of a system depend on?. DE leftfrac Stack Exchange Network. It is an extensive quantity it depends on the size of the system or on the amount of substance it contains. - 22312471 briannaregalado111 briannaregalado111 3 weeks ago Chemistry College answered What does the total thermal energy of a system depend on.
Matter can change from one state to another when thermal energy is absorbed or released. Heat is a form of energy but it is energy in transitHeat is not a property of a system. -The total number of atoms in the system.
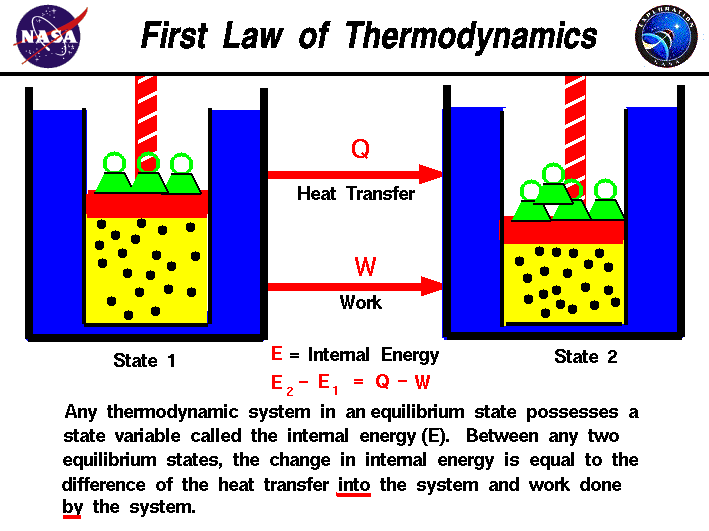
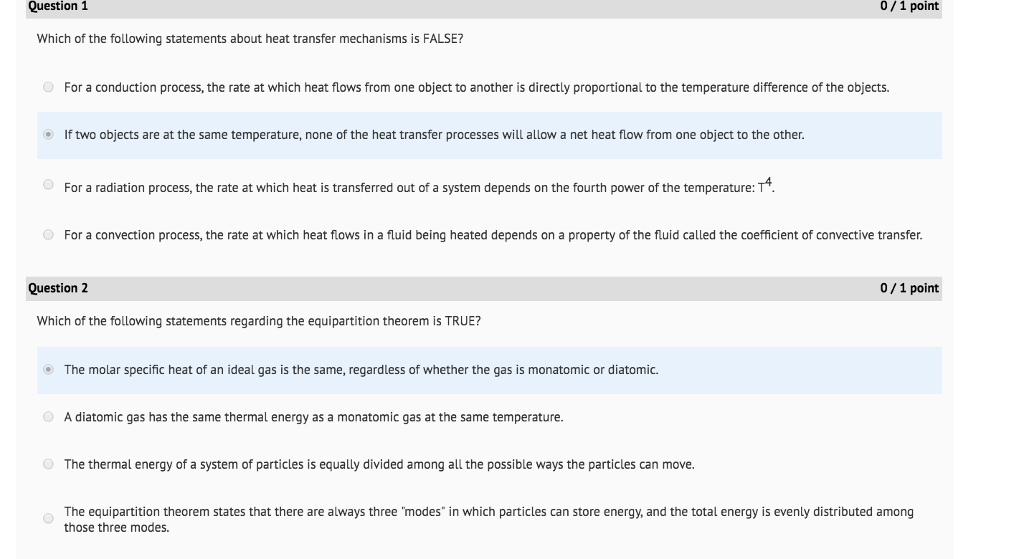
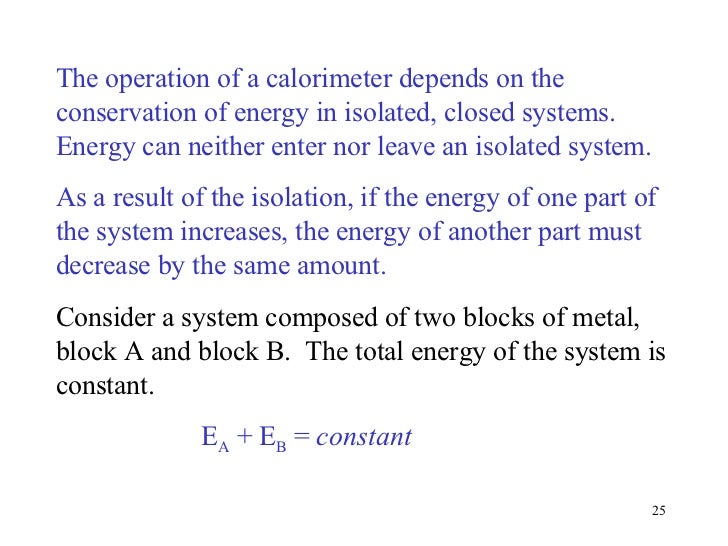
That is the total energy of a system plus its surroundings is constant which must be true if energy is conserved. Thermal energy transfer We have discussed heat flow in terms of what it does to a system but we have not examined how it happens. The total thermal energy of a system depends on its temperature and what other factors.
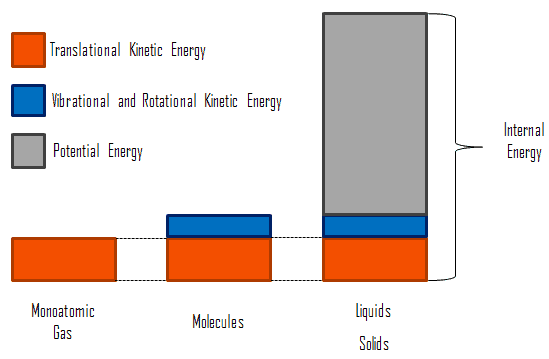
The thermal energy of an object depends on three things. When released theyll want to fly away from each other and head out to infinity. Like potential energy the internal energy can be stored in the system.
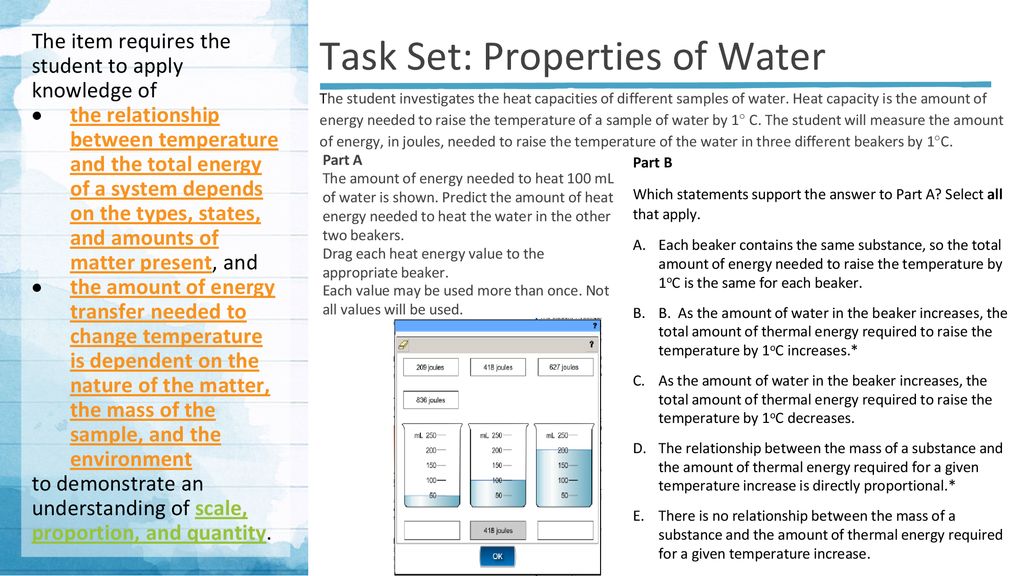
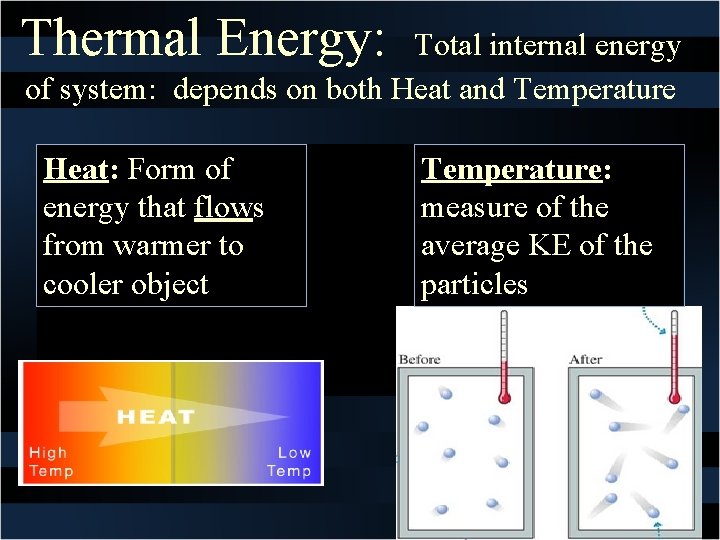
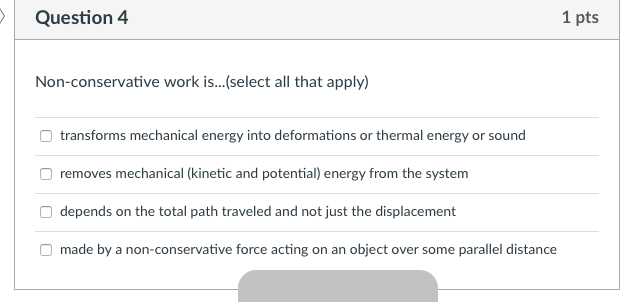
The state of a system is a complete description of a system at a given time including its temperature and pressure the amount of matter it contains its chemical composition and the physical state of the matter. The mechanical energy of a system is the sum of its potential energy and kinetic energy of the objects. A substances total thermal energy depends on its temperature number of atoms and physical state.
What mass of carbon dioxide is produced from the complete combustion of 45010. Temperature is the average kinetic energy of the molecules. Change in thermal energy mass specific heat capacity.
The particles at the interface pass the energy along by. 3 Show answers Another question on Chemistry.
What does the total thermal energy of a system depend on.
While thermal energy refers to the total energy of all the molecules within the object heat is the amount of energy flowing from one body to another spontaneously due to their temperature difference. Temperature is a measure of the average translational kinetic energy per molecule in the object while heat is the the thermal energy transferred from one thing to another due to a temperature difference. As pure substances go through a phase change their temperature remains unchanged though thermal energy continues to be added toreleased from the system. What Youll Learn what temperature is how thermal energy depends on temperature how thermal energy and heat are related calculate the change in thermal energy 1 Temperature and Heat 4A 5A Before You Read You wake up in the morning and get out of bed. Thermal energy transfer We have discussed heat flow in terms of what it does to a system but we have not examined how it happens. Heat and Matter DRAFT. The mechanical energy of a system is the sum of its potential energy and kinetic energy of the objects. In thermodynamics internal energy also called the thermal energy is defined as the energy associated with microscopic forms of energy. Energy transferred due to the temperature difference between two objects.
The SI unit of internal energy is the joule J. In thermodynamics internal energy also called the thermal energy is defined as the energy associated with microscopic forms of energy. What does the total thermal energy of a system depend on. The amount of thermal energy. The total thermal energy of a system depends on its temperature and what other factors. Heat is a form of energy but it is energy in transitHeat is not a property of a system. As a function of state the internal energy does not depend on the manner or on the path through intermediate steps by which the system arrived at its state.























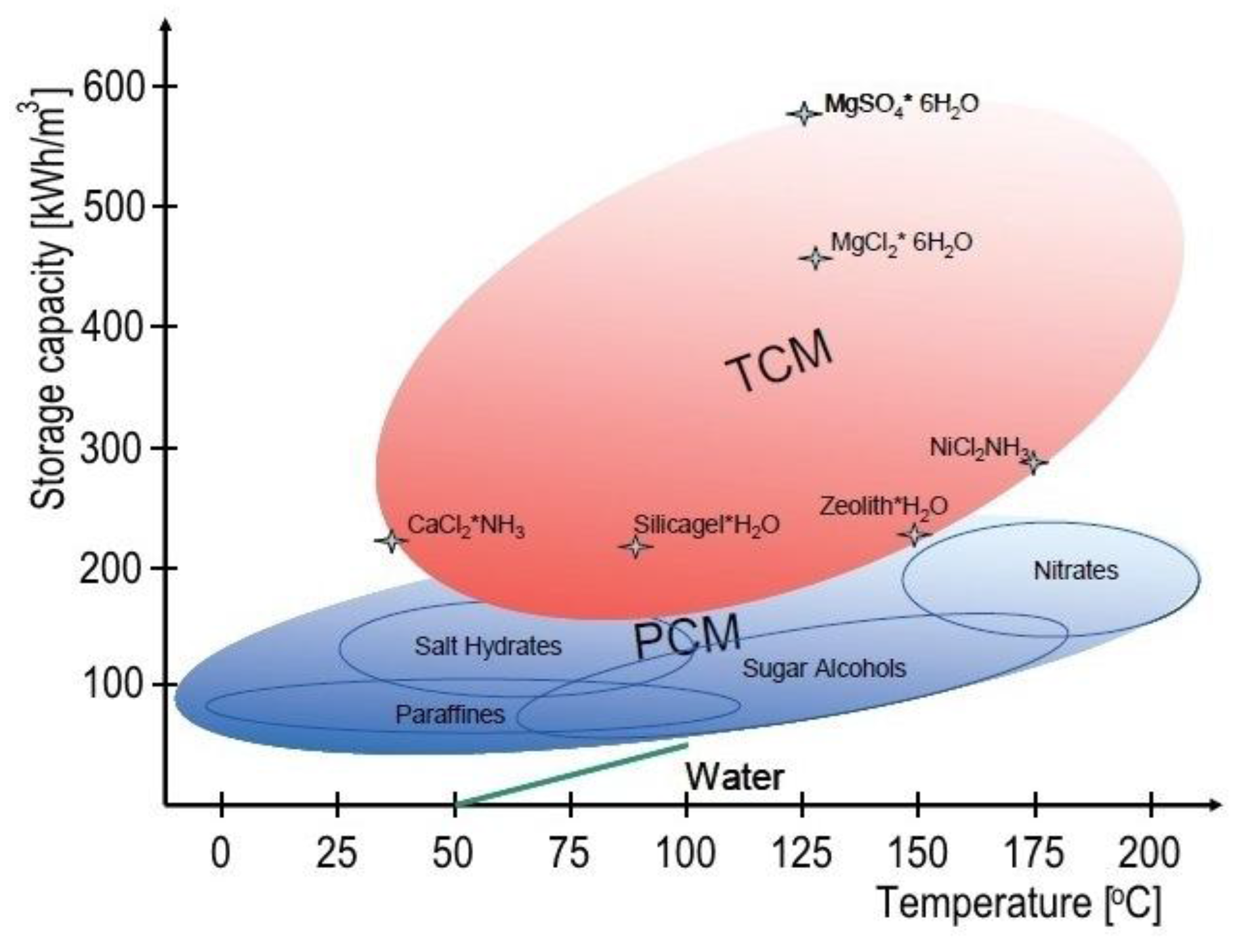





Post a Comment for "What Does The Total Thermal Energy Of A System Depend On?"